Comparing 4 vendors in Vascular Access Devices Startups across 0 criteria.
The Vascular Access Devices market is undergoing rapid transformation, driven by technological advancements and rising global demand for improved healthcare solutions. These devices, essential for administering medications, fluids, nutrition, and performing diagnostic or therapeutic procedures, include central venous catheters, peripheral catheters, and dialysis catheters. Their versatility makes them vital in various clinical settings. Increased usage is fueled by the growing prevalence of chronic diseases, more frequent surgical procedures, and the shift toward home healthcare. Additionally, an aging population and rising lifestyle-related illnesses are further propelling market growth.
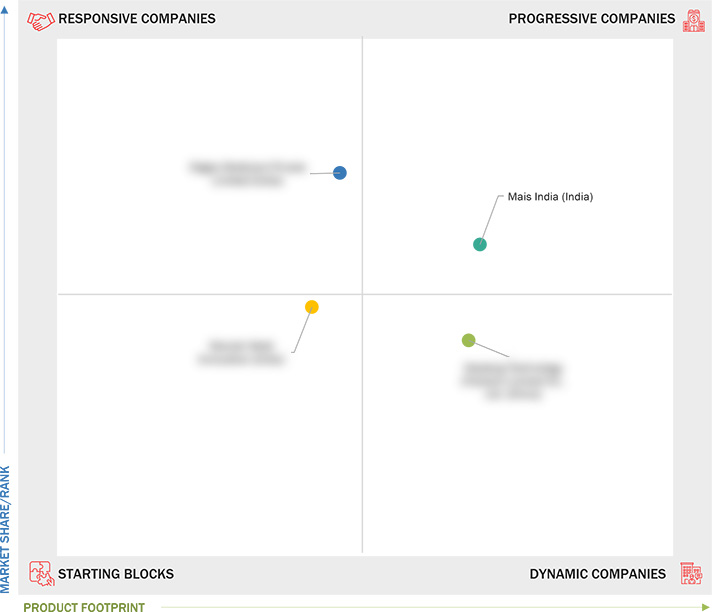
Market Leadership Quadrant
1.1 Study Objectives
1.2 Market Definition
1.3 Study Scope
1.3.1 Segments Considered & Geographical Spread
1.3.2 Inclusions & Exclusions
1.3.3 Years Considered
1.3.4 Currency Considered
1.4 Stakeholders
1.5 Summary of Changes
2.1 Introduction
2.2 Market Dynamics
2.2.1 Drivers
2.2.1.1 Growing prevalence of lifestyle diseases
2.2.1.2 Increasing popularity of chemotherapy procedures among
cancer patients
2.2.1.3 Rising Use of vascular access devices among pediatric
patients
2.2.2 Restraints
2.2.2.1 High cost of installation and maintenance of vascular
access devices
2.2.2.2 Rising product recalls and failures
2.2.3 Opportunities
2.2.3.1 Technological advancements
2.2.3.2 Increasing number of hospitals and expanding patient pool
2.2.4 Challenges
2.2.4.1 Shortage of skilled healthcare professionals
2.2.4.2 Risks associated with Use of vascular access devices
2.3 Technology Analysis
2.3.1 Key Technologies
2.3.1.1 Imaging and navigation technologies
2.3.1.2 Catheter securement and stabilization technologies
2.3.2 Complementary Technologies
2.3.2.1 AI and ML
2.3.3 Adjacent Technologies
2.3.3.1 Infection control technologies
2.4 Industry Trends
2.4.1 Minimally Invasive Vascular Access Procedures
2.4.2 Innovations in Catheter Materials and Coatings
2.5 Value Chain Analysis
2.6 Ecosystem Analysis
2.6.1 Role in Ecosystem
2.7 Supply Chain Analysis
2.8 Trade Analysis
2.8.1 Import Data for HS Code 901839
2.8.2 Export Data for HS Code 901839
2.9 Porter’s Five Forces Analysis
2.9.1 Threat of New Entrants
2.9.2 Threat of Substitutes
2.9.3 Bargaining Power of Buyers
2.9.4 Bargaining Power of Suppliers
2.9.5 Intensity of Competitive Rivalry
2.10 Patent Analysis
2.10.1 Insights: Jurisdiction and Top Applicant Analysis
2.11 Key Conferences & Events, 2025–2026
2.12 Adjacent Market Analysis
2.12.1 Vascular Closure Devices Market
2.13 Unmet Needs/End-User Expectations
2.14 Trends/Disruptions Impacting Customer’s Business
2.15 Investment & Funding Scenario
2.16 Impact of AI/Gen AI on Vascular Access Devices Market
3.1 Introduction
3.2 Key Player Strategies/Right to Win
3.2.1 Overview of Strategies Adopted By Key Players in Vascular Access
Devices Market
3.3 Revenue Analysis, 2019–2023
3.4 Market Share Analysis, 2023
3.5 Company Evaluation Matrix: Startups/SMEs, 2023
3.5.1 Progressive Companies
3.5.2 Responsive Companies
3.5.3 Dynamic Companies
3.5.4 Starting Blocks
3.5.5 Competitive Benchmarking: Startups/SMEs, 2023
3.5.5.1 Detailed list of key startup/SME players
3.5.5.2 Competitive benchmarking of key emerging players/startups
3.6 Company Valuation & Financial Metrics
3.6.1 Financial Metrics
3.6.2 Company Valuation
3.7 Brand/Product Comparison
3.8 Competitive Scenario
3.8.1 Product Launches & Approvals
3.8.2 Deals
3.8.3 Other Developments
4.1 EDGES MEDICARE PRIVATE LIMITED
4.1.1 Business overview
4.1.2 Products/Solutions/Services offered
4.1.3 Recent developments
4.2 MAIS INDIA (INDIA)
4.2.1 Business overview
4.2.2 Products/Solutions/Services offered
4.2.3 Recent developments
4.3 MANISH MEDI INNOVATION (INDIA)
4.3.1 Business overview
4.3.2 Products/Solutions/Services offered
4.3.3 Recent developments
4.4 HAOLANG TECHNOLOGY (FOSHAN) LIMITED CO., LTD. (CHINA)
4.4.1 Business overview
4.4.2 Products/Solutions/Services offered
4.4.3 Recent developments